
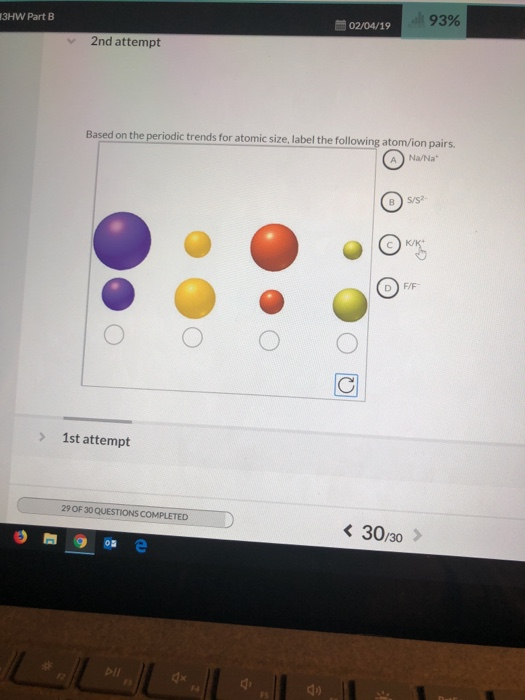
Which particle has the larger radius in each atom/ion pair? Circle your answer. Examine the trend in atomic radius from left to right across a period by clicking on all the elements in the 2 nd period. As you go down a family the atomic size increases b/c more shells are filled, increasing the distance between the outer shell to the nucleus.

Explain the relationship between the relative size of an ion to its neutral atom and the charge on the ions. What is the trend for atomic size as one proceeds down a family and explain the reason. What is the difference between a cation and an anion? 8. Explain why you see this trend as you move across a period. What is the periodic trend for atomic size from top to bottom in a group. Which atom in each pair has the larger atomic radius a) Li or K b) Ca or Ni c) Ga or B d) O or C e) Cl or Br f) Be or Ba g) Si or S h) Fe or Au. Does the same trend hold true Is this the trend you predicted Yes. Use the periodic table and your knowledge of periodic trends to answer the following questions. Choose a couple more periods (excluding 1, 6, and 7). There are some outliers (not addressed in this worksheet). Arrange the following in order of increasing atomic radius atomic radius. It trends downward so the atomic radius gets smaller across a period. Why do atoms get larger as you move down a group? 5. Why do atoms get smaller as you move left to right in a period? 4. As you move from left to right across a period on the periodic table the size of an atom will. What is the periodic trend for atomic size from top to bottom in a group? From left to right in a period? 3. Periodic, Trend, Ionization, Element, Atomic, Atom, Definition, Electronicity, Electron, Leaf, Trends, Leaf 3-3 Periodic Trends - Ms. a) Li or K b) Ca or Ni c) Ga or B d) O or C e) Cl or Br f) Be or Ba g) Si or S h) Fe or Au 2. Which atom in each pair has the larger atomic radius? Circle your answer.
UNIT PERIODIC TRENDS ATOMIC SIZE TREND WORKSHEET 2 ANSWERS PDF
Download Periodic Trends Worksheet and more Chemistry Lecture notes in PDF only on Docsity!Chemistry ICP for Jan 22: Periodic Trends Use the periodic table, charts, and your knowledge of periodic trends to answer the following questions.


 0 kommentar(er)
0 kommentar(er)
